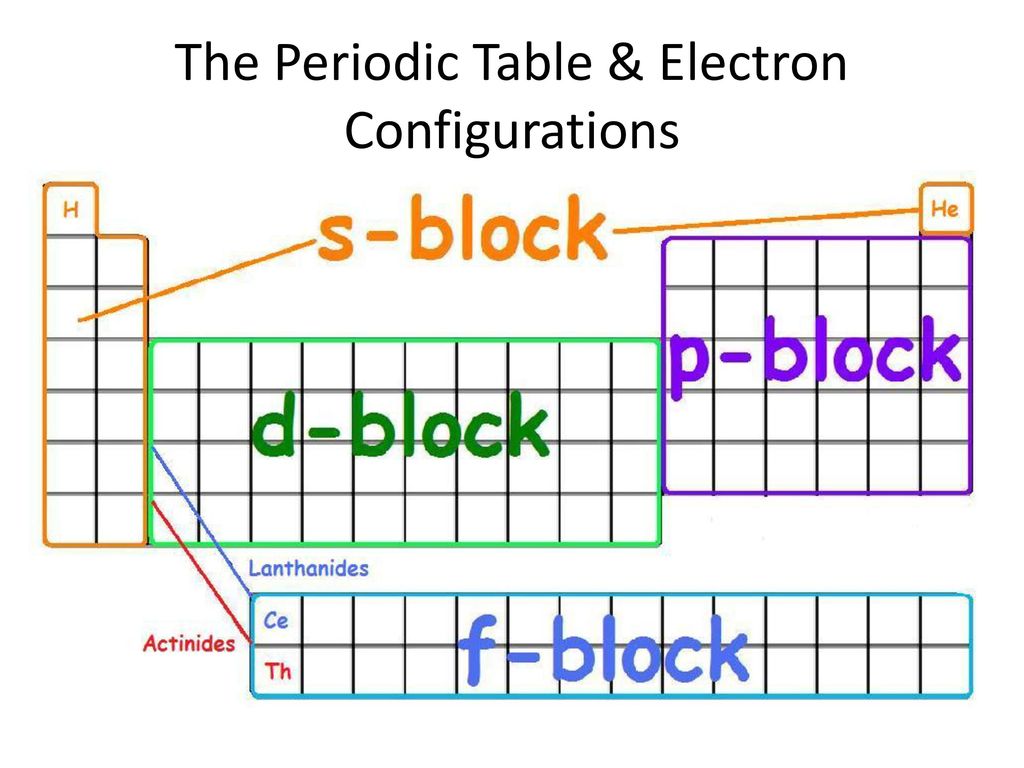
S D P Periodic Table

Learn what element blocks are and their properties and characteristics.
S d p periodic table. But some have lot of oxidation numbers. The labels s p d and f blocks of the periodic table refer to the subshell that is being filled with electrons. Chemical elements alphabetically listed the elements of the periodic table sorted by name in an alphabetical list. Oxidation numbers of elements in periodic table s p d blocks.
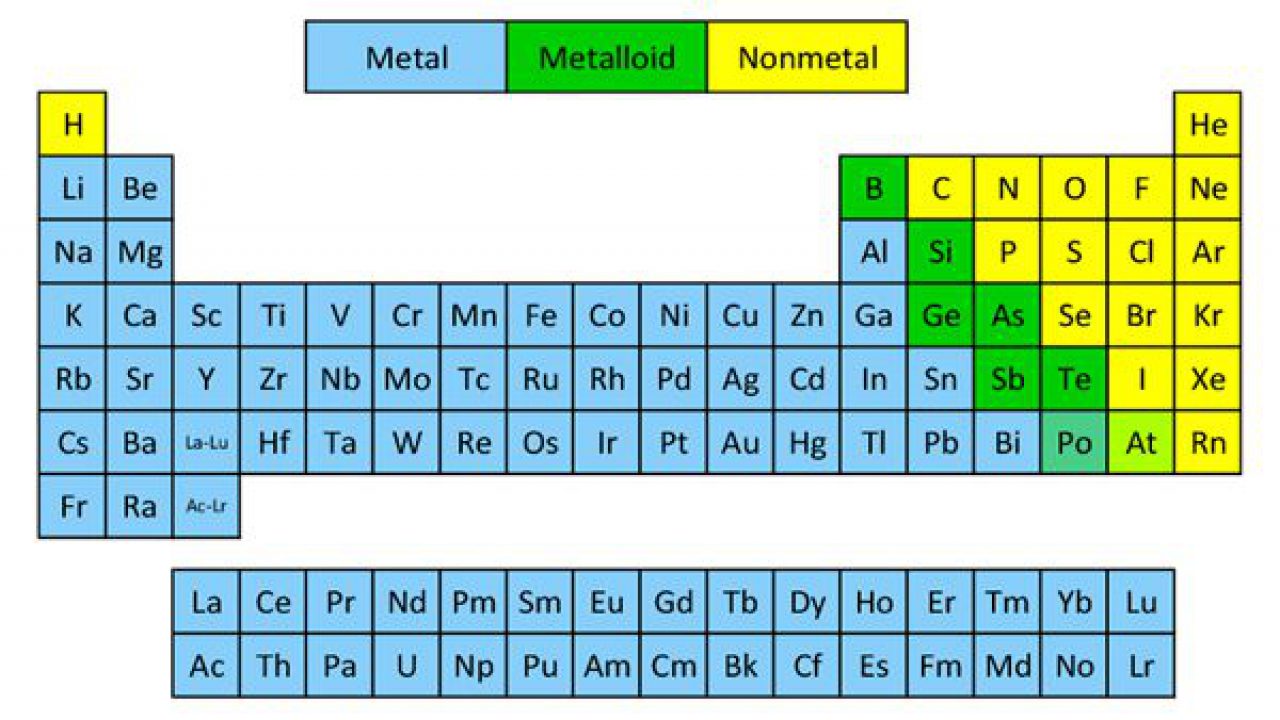
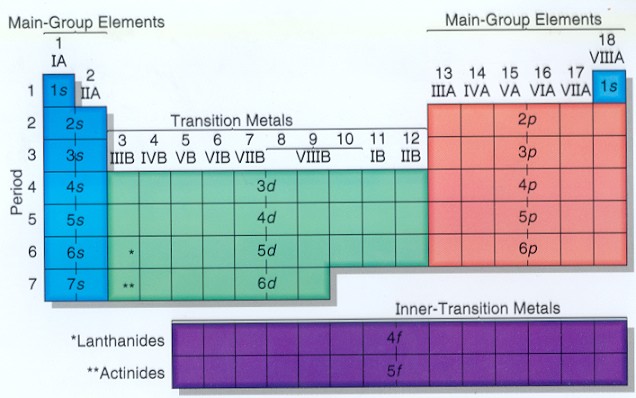
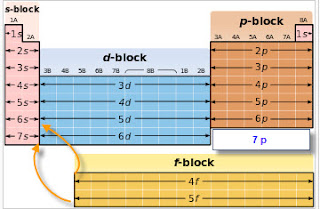
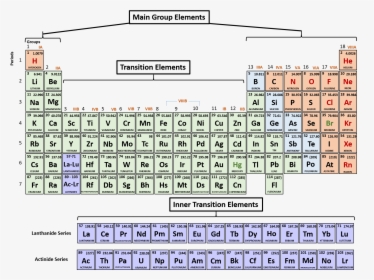
Click on any element s name for further chemical properties environmental data or health effects. Visualize trends 3d orbitals isotopes and mix compounds. Modern periodic law moseley the english physicist showed that atomic number is more fundamental property of an element than its atomic mass. The periodic table of elements can be organized by blocks s p d f g.
These are s p d and f blocks. Group 1 elements occur at the beginning of a new row period of the periodic table. General electronic configuration of s block elements. In sodium compounds sodium only forms 1 oxidation number.
Therefore the position of an element in the periodic table depends on its atomic number than its atomic mass. Helium belongs to s block but its positioning with in the p block along with other group 18 elements is justified because it has a completely filled valence shell and thus exhibits properties characteristic of other noble gases. Some elements in the periodic table have only one oxidation number or two oxidation numbers. Oxidation number of element in a compound can be positive or negative or may be zero.
Relationship between s p d and f blocks and electronic configuration. 6 there are 14 s block elements in the periodic table. Alphabetical list of chemical elements periodic table chart. This list contains the 118 elements of chemistry.
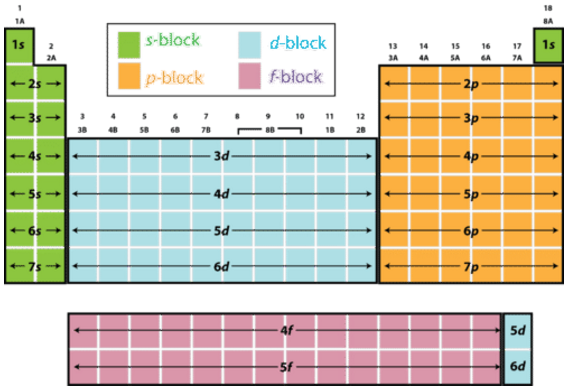
Division of periodic table into various blocks. The division of elements into blocks is primarily based upon their electronic configuration as shown in fig. The highest energy level valence shell contains only 1 electron in an s. Interactive periodic table showing names electrons and oxidation states.
Ns 1 2 where n 2 7.
/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)


/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)